![]() MONIKA OSIŃSKA-JAROSZUK1, MAGDALENA JASZEK1, JUSTYNA SULEJ1, DAWID STEFANIUK1, MONIKA URBANIAK2, MAREK SIWULSKI3 and GRZEGORZ JANUSZ1*
MONIKA OSIŃSKA-JAROSZUK1, MAGDALENA JASZEK1, JUSTYNA SULEJ1, DAWID STEFANIUK1, MONIKA URBANIAK2, MAREK SIWULSKI3 and GRZEGORZ JANUSZ1*
1Biochemistry Department, Maria Curie-Sklodowska University, Lublin, Poland
2Department of Pathogen Genetics and Plant Resistance, The Institute of Plant Genetics of the Polish Academy of Sciences, Poznań, Poland
3Department of Vegetable Crops, Poznan University of Life Sciences, Poznań, Poland
*Corresponding author: G. Janusz, Biochemistry Department, Maria Curie-Sklodowska University, Lublin, Poland; e-mail: gjanusz@poczta.umcs.lublin.pl.
Submitted 17 June 2014, revised 30 April 2015, accepted 11 February 2016
DOI: 10.5604/17331331.1215609
Abstract
The present study examined Polish strains of Flamulina velutipes as a potential source of nutraceuticals and found that their nutritional value is dependent on the fruiting bodies gathering time. To prove the above hypothesis protein, carbohydrate and phenolic substances concentration were determined. Moreover, catalase, superoxide dismutase, cellobiose dehydrogenase activities were assayed. In order to prove the healing properties of Enoki fruiting bodies the obtained extracts were tested for antioxidant and bacteriostatic abilities. We have proved that Polish F. velutipes fruiting bodies may be a rich source of antioxidants and that they are capable of inhibiting Staphylococcus aureus growth.
Key words: Flammulina velutipes, antibacterial activities, antioxidative activities, fruiting body, phenolic compounds
Introduction
It is presumed that there are more than 14000 species of mushrooms including at least 2000 with various degrees of edibility, of which about 200 edible mushrooms are wild species (Chang, 1987; 2008; Kalac, 2013; Zhang et al., 2013a). During thousands of years, mushrooms have been valued throughout the world as both food and medicine due to their taste and nutritional value as well as healing properties (Wright, 2004; Adebayo et al., 2014). Over time, the importance of mushrooms in human diet has led to the cultivation of certain species. It seems that Auricularia auricularis (the wood ear mushroom) was the first fungal species cultivated in China around AD 600 (Kues and Liu, 2000). Over the centuries, the number of cultivated species has been growing together with various applications not only in cuisine but also in medicine. Nowadays, mushrooms are important ingredients of baked dishes (ex. Pleurotus sp., Ganoderma sp., Lentinula sp.), soups (Lentinula sp., Agaricus bisporus), or drinks (Ganoderma lucidum). Moreover, they are used instead of Saccharomyces to produce alcohol (e.g. Agaricus blazei, Flammulina velutipes, Pleurotus ostreatus) (Moon and Lo, 2013). To date, the reported healing properties of edible fungi encompass anticancer (Ganoderma sp. (Wu et al., 2013a), Lentinula edodes (Cao et al., 2013)), antiviral (Ganoderma pfeifferi (Niedermeyer et al., 2005), Pleurotus ostreatus (Santoyo et al., 2012)), immunomodulatory (Grifola frondosa (Wu et al., 2013b)), anti-diabetic (A. blazei (Di Naso et al., 2010)), and cardiovascular (Volvariella volvacea (Chiu et al., 1995)) activity. Moreover, a number of edible fungal species possess antioxidant properties (G. lucidum, Hericium erinaceus) (Deepalakshmi et al., 2013; Han et al., 2013). Recently, an explosion of investigations describing bioactive compounds from fungi has been observed, and with every study, the number of isolated and characterized compounds is growing. Each paper brings to light new healing properties of fungal glucans (Wiater et al., 2012), proteins (Jaszek et al., 2013), enzymes (Xu et al., 2011) and other (Mahmood et al., 2010; Ma et al., 2011). Enoki (F. velutipes) is one of the most valuable species in Asian countries with little importance in Europe. Despite its culinary values, cultivation and merchandise thereof in certain European countries is even illegal. At the same time, recent papers have proved that F. velutipes possess anticancer, immunomodulatory, anti-inflammatory, and antioxidant activities (Chang et al., 2013; Zhang et al., 2013b; Gunawardena et al., 2014). Beside its obvious medicinal properties, Enoki has been confirmed as a source of laccase, proteases, sterols, endo-β-1,3-galactanase, or asparaginase (Eisele et al., 2011; Kotake et al., 2011; Iketani et al., 2013; Kim et al., 2013; Tong et al., 2014). Despite laboratory experiments with Enoki mycelium, little is known about the nutritional composition and medicinal properties of wild Flammulina fruiting bodies (Ergonul et al., 2013; Lin et al., 2013) and even less about cultivated ones (Cai et al., 2013). Given the dynamics of production of bioactive compounds by fungal mycelium, it is obvious that the same effect may be observed in its fruiting bodies. To retain the maximum medicinal value of consumed mushrooms, they should be harvested at the time of their maximum activity. The aim of the paper was to prove not only the medicinal potential of new F. velutipes strains isolated in Polish ecological regions, but also to show that fruiting bodies grown in mushroom farms should be harvested at an appropriate time to preserve their maximum healing values.
Experimental
Materials and Methods
Strains, medium and growth processing. F. velutipes strains Fv4, Fv10, and Fv11 were obtained from the culture collection of the Department of Vegetable Crops, Poznań University of Life Sciences. The strains were identified by ITS sequencing previously (Janusz et al., 2014). Pure cultures were isolated by cutting out a piece of trama from the inner part of carpophores and placing it onto 20 g/kg malt agar medium in a Petri dish. Stock cultures of the strains were grown for 7 days at 30°C on potato dextrose agar. They were stored at 4°C and subcultured every month. The cultures were used for producing the grain spawn (wheat grain) by a convenient method. The prepared spawn was stored at 4°C until it was used for inoculation.
Fruiting conditions. Cultivation of F. velutipes fruiting bodies was carried out at the Department of Vegetable Crops of Poznan University of Life Sciences. The applied substrate was a mixture of oak and beech sawdust (1:1, v/v). The above-mentioned sawdust mixture was enriched by addition of wheat bran in the amount of 200 g/kg DM (Dry Matter) of the sawdust as well as saccharose and gypsum in the amount of 10 g/kg. The experimental substrate was wetted with distilled water to reach a moisture content of 650 g/kg and used to fill polypropylene sacks equipped with a micro-filter. Each sack contained 2.5 kg wet substrate. The substrate was subjected to sterilization at the temperature of 121°C for 1.5 h and, after cooling it down to the temperature of 25°C, it was inoculated with the mycelium of the F. velutipes strains. Incubation was conducted in darkness at the temperature of 25°C and relative air humidity of 80–85% until the entire substrate was overgrown by mycelium. Next, the sacks were transferred to the cultivation facility and the foil was cut off in their upper part directly over the surface of the substrate. Throughout the trial, the temperature in the facility was maintained at 14–15°C and relative air humidity at 85–90%. The facility was additionally lit with fluorescent light (day–light) of 4500 cd intensity and ventilated to keep the CO2 concentration below 1 lm3. The fruiting bodies were harvested depending on the strain growth to achieve the same stage of growth (Fig. 1). Therefore, for strain Fv4 time zero means 43 days, Fv10 – 36 days and for Fv11 – 51 days. Next, the fruiting bodies were harvested at 0, 1, 2, 3, and 4 days.

Preparation of extracts from the fruiting body biomass. Fungal fruiting bodies were harvested periodically during 96 hours after the beginning of the fruiting (at 0, 1, 2, 3, and 4 days). The fruiting body biomass was homogenized in distilled water in the proportion 1:1 with a glass Potter homogenizer at 4°C. After centrifugation (15 min, 10000 x g), portions of the crude supernatant were frozen and used for the conducted experiments.
Analytical methods
Determination of carbohydrates, proteins, and phenolic compounds. The carbohydrate concentration of the extracts isolated from the fruiting bodies was measured by the phenol-sulfuric acid assay according to DuBois et al. (1956) with D-glucose as a standard. The reducing sugar was determined by the Somogyi-Nelson methods based on the procedure described by Hope and Burns (1987) with some modifications. The protein content was analyzed according to the Bradford (1976) method using bovine serum albumin (BSA) as a standard. In addition, the phenolic compounds in the extracts from the fruiting body were quantified by the DASA test (Malarczyk, 1989). The changes in absorbance were measured at 500 nm and compared with the standard curve of vanillic acids.
Laccase activity assay. Laccase activity was measured using syringaldazine (4-hydroxy, 3,5-dimetoxybenzaldehyde) as a reaction substrate (Leonowicz and Grzywnowicz, 1981). The catalytic activity of enzyme was expressed in nanokatals per milligram of protein.
Cellobiose dehydrogenase activity assay. Cellobiose dehydrogenase (CDH) activity was measured by monitoring the change in absorbance of the two-electron acceptor 2,6-dichloroindophenol (DCIP) (Sigma Chemical Co., St. Louis, MO, USA) at 520 nm (ε520 = 6.8 mM−1cm−1), pH 4.5, and 30°C using a Shimadzu UV160A (Shimadzu, Tokyo, Japan) spectrophotometer. The reaction mixture consisted of DCIP (50 µl, 3 mM in water containing 10% v/v ethanol), sodium fluoride (50 µl, 80 mM in water as an inhibitor for potentially present laccases), lactose (100 µl, 300 mM in 100 mM sodium acetate buffer, pH 4.5), 700 µl of the same buffer, and 100 µl of the enzyme solution appropriately diluted in a 1 ml glass microcuvette. The reaction was started by addition of the enzyme and the decrease in absorbance was monitored during the first 60 s. The final enzyme activity was expressed as nkat per liter (Baminger et al., 2001; Karapetyan et al., 2006).
Assay of the relative level of superoxide anion radicals (SOR). The SOR level was estimated according to the method for rapid detection of superoxide anion presence in fungal material (Pazdzioch-Czochra et al., 2003). The spectrophotometric measurements were based on the detection of superoxide-induced formation of formazan from nitrotetrazolium blue (NBT) under alkaline conditions, as described previously (Jaszek et al., 2006). The alkaline conditions were introduced to prevent precipitation of formazan for about 40 min.
Antioxidant activity assays
ABTS Radical-Scavenging Test. The ABTS radical-scavenging ability of the extracts isolated from the fruiting bodies were recorded according to the procedure of Re et al. (1999) with some modification. For detection of the antioxidant capacity, 10 µl of the investigated compounds at concentrations ranging from 15 to 500 mg/ml were mixed with 990 µl of the ABTS radical solution. The determination of absorbance stability in the range of 1 to 15 minutes was checked for both mushroom preparations. The stability of absorbance of the samples was observed after 6 minutes of incubation. Accordingly, this time (60 s) was then used in the assays. The percentage of reduction of ABTS oxidation was calculated by the presented formula:
ABTS+ scavenging effect (%) = [(A0-A1)/A0] x 100
where A0 means the absorbance of the control samples and A1 stands for the absorbance at 734 nm of the investigated compounds/standards.
DPPH Free Radical-Scavenging Test. The DPPH free radical-scavenging ability of the extracts isolated from the fruiting bodieswas determined according to the method described by Paduch et al. (2008). The analysed compounds (0.1 ml) at concentrations ranging from 6.25 to 600 mg/ml were added to 0.1 ml of a DPPH. solution (0.2 mg/ml in ethanol). Trolox and ascorbic acid (standards with strong antioxidant activities) were used as positive markers. Absorbance at 515 nm was determined at room temperature after 2, 5, 10, 15, 20, and 30 min of incubation. The optimal time of incubation in the presented measurement was 10 min. The percentage of reduction of the DPPH oxidation rate was calculated according to the presented formula:
DPPH scavenging effect (%) = [A0-(As – Ac)/A0] x 100
where A0 means the absorbance of the control sample (with DPPH), and As means the absorbance of the standards or investigated compounds (with DPPH), Ac means the absorbance of the investigated compounds (without DPPH). The Trolox calibration curves for both tests were prepared for a concentration range from 15 to 500 mg/ml and EC50 values were indicated as described previously (Jaszek et al., 2013).
Analysis of the antibacterial activity of extracts from the fruiting body. The antibacterial effect of the extract isolated from the fruiting bodies was tested using Escherichia coli ATCC 25922 and S. aureus ATCC 25923 bacterial strains. Before testing, inocula of each bacterium were grown in the Luria–Bertani (LB) medium. The tested substances (100 µl) were added to 48-well sterile polystyrene plates containing 1 ml of the LB medium. The inoculum was determined by measuring the optical density and expressed as the number of cells per ml. The inocula were used in infecting doses as follows: E. coli: 1 × 104, 1 × 106 cells/ml and S. aureus: 1 × 104, 2 × 106cells/ml. During a 24-hour incubation period (at 37°C), the LB medium samples from each experiment were tested for bacterial growth by measuring the OD (optical density) on a plate reader at 660 nm.
Electrophoretic visualization of the activities of superoxide dismutase (SOD) and catalase (CAT) – enzymatic antioxidants and protein profiles. The samples of the prepared homogenate were separated by ultrafiltration using the Microcon Centrifugal Filter Units, 3000 NMWL designed by Millipore. Afterwards, 15 µg of proteins from the samples were loaded into each well of 10% native polyacrylamide gel. The gels were run at 4°C and 145 V. After separation, SOD activities were visualized based on the method of Beyer and Fridovich (1987). The CAT activity bands were visualized using ferricyanide negative staining according to the Wayne and Diaz (1986) methodology. For the detection of protein bands, Coomassie brilliant blue (R-250) staining was used.
Statistical analysis. Statistical analysis of data from independent experiments repeated three times was performed on three replicates from each treatment with Excel program Microsoft Office 2010 package and the results presented in the paper were a mean ±SD from three experiments and three repetitions (n = 9). Multiple comparisons of means were performed by the Tukey Honest Significance Post Hoc Test. P values ≤ 0.05 were considered significant for all the tests. Analysis of variance ANOVA was used to determine significance of differences between values.
Results
Chemical composition of extracts from F. velutipes fruiting bodies. The extracts from the fruiting bodies of three F. velutipes strains were evaluated in terms of the concentration of protein, total sugar, reducing sugar, and phenolic compounds. The results obtained show that the maximum concentration of the substances varied during the development of the analyzed fungal fruiting bodies. The maximum concentration of proteins (over 10 mg/g dry weight) was found in fruiting bodies harvested on the first or second day. Total sugars achieved their maximum concentration later (up to even 4 days) and reached up to 15.4 mg/g of dry weight, whereas the concentration of reducing sugars seemed to be changing during these days and the pattern was hard to be noticed. In all the analyzed strains, the maximum concentration of phenolic substances (up to 9.57 mM/g dry weight) was observed in the fruiting bodies harvested on the second or third day of fructification. It appears that strains Fv4 and Fv10 are richer in proteins than Fv11 (Table I).
Table I
Chemical composition of extracts isolated from fruiting body of F. velutipes yield of proteins, total sugars, reducing sugars and concentration of phenolic compounds. Data are mean ±SD for three experiments and three replicates (n = 9). The mean values in columns marked by the same lowercase letter are not significantly different at significance of p ≤ 0.05 determined according Tukey’s honest significance test.
| Samples | Protein
(mg/g*) |
Total sugars
(mg/g*) |
Reducing sugars (mg/g*) | Total phenolic (mM/g*) |
| Fv4/0
Fv4/1 Fv4/2 Fv4/3 Fv4/4 |
6.7±0.10c
10.9±0.10a 6.8±0.05c 5.2±0.20e 5.3±0.10e |
6.7±0.40g
6.1±0.07g 7.9±0.40f 11.4±0.30c 7.6±0.30f |
2.9±0.02b
2.6±0.06c 3.4±0.10a 3.2±0.02a 2.3±0.08d |
7.5±0.20b
9.3±1.20a 9.6±0.20a 6.2±0.10c 6.4±0.10c |
| Fv10/0
Fv10/1 Fv10/2 Fv10/3 Fv10/4 |
10.4±0.03a
6.8±0.08c 4.7±0.10f 3.3±0.01i 4.5±0.09g |
9.8±0.10e
9.8±0.02e 13.1±0.20b 8.8±0.50f 6.5±0.70g |
2.0±0.07d
1.8±0.05e 2.1±0.06d 1.3±0.10f 2.2±0.10d |
7.6±0.05b
5.9±0.20d 6.3±0.08c 4.2±0.20e 4.0±0.10e |
| Fv11/0
Fv11/1 Fv11/2 Fv11/3 Fv11/4 |
5.3±0.10e
6.1±0.10d 4.1±0.10h 4.8±0.08f 7.3±0.09b |
9.9±0.10e
15.4±0.80a 10.1±0.10d 10.6±0.05d 13.7±1.40b |
2.1±0.25d
2.5±0.03c 1.4±0.20f 2.0±0.10e 1.6±0.10f |
9.6±0.70a
8.1±0.50b 5.9±0.30d 6.6±0.04c 5.8±0.06d |
Mean values in columns with same lowercase letters are not significantly different at p ≤ 0.05.
*– g dry weight of fruiting body
Moreover, the electrophoretic profiles of Fv4 and Fv10 differ from that of Fv11 (Fig. 2). It was observed that the amount of protein contained in the fruiting bodies was decreased with the time of its development. In addition, the electrophoretic analysis confirmed the fact the highest amount of protein was observed in the fruiting bodies of strain F4. However, this strain seems to possess more total sugars than Fv4 and Fv10 (Table I).

Enzyme activities: CDH, CAT, SOD. The highest activities of cellobiose dehydrogenase (CDH), catalase (CAT), and superoxide dismutase (SOD) were observed in extracts isolated from Fv4. CDH and SOD seemed to be most active in the fruiting bodies of the strain when they were harvested on the second day. The activities of CDH were at least twice as high as in other two strains (Fig. 3).

Moreover, the maximum activity of CDH in the Fv10 fruiting bodies was observed on the same day (24 hours), whereas the highest activity in Fv11 appeared 24 hours earlier and was continuously declining. The highest activity of (CAT) was noted in the fruiting bodies of Fv4 and remained almost unchanged over the time, in contrast to Fv10 and Fv11, in which they were obviously decreasing day after day (Fig. 4A). The electrophoretic analysis of SOD activities proved existence of two clearly visible isoforms in all the analyzed strains. The highest SOD activities were observed in the Fv4 strain, contrary to those of Fv11. In all the investigated fruiting bodies, the level of SOD decreased during the fructification period (Fig. 4B). No laccase or manganese peroxidase activities were detected in all the analyzed fruiting body extracts.

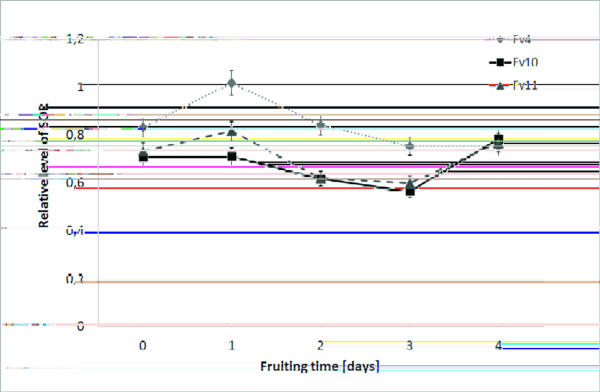
Antioxidant and antibacterial activities. To allow comparison of the antioxidant properties of the tested strains, we checked the level of superoxide anion radicals. The results obtained allow a conclusion that the highest concentration was observed in the fruiting bodies of the Fv4 strain harvested after 1 day. A similar pattern was found in the case of strain Fv11; however, the level of superoxide anion radicals was approx. 20% lower. The same peak was observed on the second day for the fruiting bodies of Fv10; however, both Fv10 and Fv11 had another maximum within 4 days, and the second peak was slightly higher for Fv10 (Fig. 5).
Since no laccase activity was detected in the analyzed extracts, we used the ABTS and DPPH method to estimate the antioxidant potential of the tested F. velutipes fruiting bodies. The results obtained showed clearly that all the strains exhibited the highest antioxidant activity during the first 24 hours. The scavenging abilities of F. velutipes fruiting body extracts assayed with the ABTS method at the concentration range of 15-500 mg/ml were between 13.6 and 90.5% for Fv4, 0.5 and 79.9% for Fv10, and 3.6 and 77.1% for Fv11 (Fig. 6).

In the case of the DPPH method, the maximum of the scavenging effect was 57.2% for Fv4, 58.3% for Fv10 and 58.1% for Fv11 (Fig. 7).

The calculation of normalized EC50 values specified the concentrations of extracts isolated from the fruiting bodies of F. velutipes that are able to scavenge 50% of free radicals present in the tested mixture (Table II).
Table II
EC50 values (half of the maximum scavenging effect) of extracts isolated from F. velutipes fruiting body submerged cultures with trolox as control. Data are mean ±SD for three experiments and three replicates (n = 9). EC50 > 500 mg/ml cannot be calculated from the graphs. The mean values in columns marked by the same small lowercase and in rows marked by the same uppercase letter are not significantly different at significance of p ≤ 0.05 determined according Tukey’s honest significance test (HSD).
| Samples | EC50(mg/ml) | |
| DPPH method | ABTS method | |
| Fv4/0
Fv4/1 Fv4/2 Fv4/3 Fv4/4 |
236.2±1.1e
246.1±1.0g 206.9±0.9h 267.6±1.2f >500 |
57.9±0.6k
56.9±0.5k 70.2±0.6j 107.1±0.9i 112.2±0.0h |
| Fv10/0
Fv10/1 Fv10/2 Fv10/3 Fv10/4 |
>500a
>500a 237.1±0.9e >500a >500a |
125.0±0.6g
130.2±0.5h 191.7±0.6d 221.6±0.7c 315.6±0.9a |
| Fv11/0
Fv11/1 Fv11/2 Fv11/3 Fv11/4 |
452.1±2.1a
430.2±2.0b 437.0±1.9c 398.0±2.0d >500a |
121.3±0.5l
155.3±0.4f 178.8±0.5d 125.0±0.6g 261.1±0.8b |
| Trolox | 8.1±1.0h | 8.04±1.1m |
Mean values in columns with same lowercase letters are not significantly different at p ≤ 0.05.
ABTS – 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)
DPPH – di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium
The antioxidant activity of the analyzed fungal extracts seemed to be increasing together with their concentration, achieving a plateau at 250 g/l. Bearing in mind that antioxidant properties of fungal extracts may be related to antibacterial potential, we tested their ability to inhibit growth of S. aureus. All the F. velutipes fruiting bodies harvested during the first two days appeared to inhibit growth of the mentioned bacterial strain, however, to a different extent. The highest antibacterial activities were detected in the extracts from Fv11, which were even capable of complete inhibition (OD660nm = 0) of bacterial growth introduced as inoculum of 1 x 106 cells/ml (Table III). Strain Fv10 appeared to be slightly less active, as it inhibited S. aureus growth introduced as lower inoculum (1 x 104 cells/ml).
Table III
Optical density (OD 660 nm) measurement of E. coli and S. aureus introduced as 1 x 104 cells/ml and incubated 1 day in LB medium at 37°C in the presence of extracts isolated from fruiting body of F. velutipes. Data are mean ±SD for three experiments and three replicates (n = 9). The mean values in columns marked by the same lowercase letter are not significantly different at significance of p ≤ 0.05 determined according Tukey’s Honest Significance test.
| Samples | OD at 660 nm | |||
| E.coli | S. aureus | |||
| 1 x 104 /ml | 1 x 106 /ml | 1 x 104 /ml | 1 x 106 /ml | |
| Fv4/0
Fv4/1 Fv4/2 Fv4/3 Fv4/4 |
0.4±0.02dA
0.6±0.02bA 0.3±0.09eC 0.3±0.01eB 0.5±0.03cB |
0.4±0.02dA
0.5±0.02cB 0.4±0.03dB 0.4±0.04cA 0.5±0.04cB |
0.1±0.02fB
1.1±0.03aC 0.7±0.02bA 0.2±0.01eC 0.4±0.02cC |
0.1±0.03fB
1.1±0.02aC 0.1±0.01fD 0.2±0.02eC 0.7±0.03bA |
| Fv10/0
Fv10/1 Fv10/2 Fv10/3 Fv10/4 |
0.4±0.02dB
0.4±0.05dB 0.7±0.01aA 0.5±0.02cB 0.6±0.03bA |
0.5±0.01 d A
0.5±0.03 d A 0.7±0.02a A 0.6±0.05 b A 0.5±0.02c B |
0.0gD
0.0gD 0.0gC 0.0gD 0.4±0.02cC |
0.1±0.02fC
0.1±0.03fC 0.2±0.01eB 0.2±0.02eC 0.5±0.04cB |
| Fv11/0
Fv11/1 Fv11/2 Fv11/3 Fv11/4 |
0.4±0.01dB
0.5±0.02cA 0.4±0.03dB 0.4±0.03dB 0.5±0.02cB |
0.6±0.05b A
0.5±0.02c A 0.5±0.04 c A 0.5±0.02c A 0.6±0.09 a B |
0.0gC
0.0gB 0.0gD 0.1±0.01fD 0.3±0.01dC |
0.0gC
0.0gB 0.2±0.01eC 0.2±0.01eC 0.3±0.02dC |
Mean values in columns with same lowercase letters are not significantly different at p ≤ 0.05.
Mean values in rows with same uppercase letters are not significantly different at p ≤ 0.05.
OD – optical density
Discussion
In recent years, an explosion in interest in healthy nutrition has been observed. Many products originating from plants or animals are labeled as ecological or good for human health. Nowadays, food products are carefully examined with respect to composition, nutritional values and finally possible medicinal properties/activities. Recent advances in biotechnology allowed introducing a number of products, including microorganisms or the result of cultivation thereof, to markets all over the world. Among them, fungi form a large group that not only is crucial for brewery products but also their importance in medicine is increasing, as indicated by many papers describing new fungal compounds or their application. In the beginning, mushrooms were valued for their flavor in preparation of dishes; nowadays, they are rather regarded as a source of nutraceuticals. Recent analyses have proved that mushrooms are important in our diet due to the high protein and low fat content (Barros et al., 2008); moreover, a number of papers have evidenced their value as antimicrobial, antioxidant, and anticancer compounds (Janes et al., 2006; Moradali et al., 2007; Karaman et al., 2010; Lemieszek and Rzeski, 2012). The results of our experiments support the findings that F. velutipes fruiting bodies may be a source of nutraceuticals in human diet. The paper presents not only three newly isolated Enoki strains but also proves that the harvesting time of fruiting bodies may be important for the level of bioactive compounds. Focusing on the nutraceutical value of Enoki, it should be underlined that the highest protein and carbohydrate concentration is observed in fruiting bodies harvested during the first days. However, there are species that contain more proteins or carbohydrates (Barros et al., 2008); nevertheless, high nutrient value tends to be observed in young fruiting bodies (Barros et al., 2007a; 2007b). Cheung et al. (2003) proved that the total phenolic compounds were responsible for the antioxidant properties of extracts from wild growing mushrooms. In our experiments, the highest total phenolic compound content in early harvested fruiting bodies support this hypothesis; however, the differences among the strains suggest that more compounds may be engaged in the F. velutipes antioxidant properties. Emerging information related to antioxidant properties of fungal cellobiose dehydrogenase (Nyanhongo et al., 2013; Sulej et al., 2013) we showed CDH activities in F. velutipes fruiting bodies in contrary to manganese peroxidase and laccase, which were not detected. To our knowledge, CDH activity has not been previously demonstrated in Enoki fruiting bodies; however, the role of this enzyme was proved to be important in pigmentation of Pycnoporus cinnabarinus (Temp and Eggert, 1999). Since we have found a correlation between cellobiose dehydrogenase (Fig. 3) and the scavenging effect (Table II) of fungal extracts, the results obtained may support CDH involvement in the antioxidant properties F. velutipes. Besides this cellulose-degrading enzyme, two other key antioxidant enzymes with high activities were found in the early harvested Enoki fruiting bodies. Both catalase and superoxide dismutase are produced by many organisms to prevent free radical damage (Garcia et al., 2003; Mau et al., 2004; Rahman, 2007). Ma et al. (2014) have proved that activities of catalase and SOD are dependent on hydratation of fruiting bodies in Auricularia auricula-judae; moreover, SOD is the most efficient enzyme scavenging superoxide anion radicals (SOR). It is possible that reactions catalyzed by SOD and catalase are responsible for decreasing the superoxide anion radical concentration. Barros et al. (2007a; 2007b) suggested that the decrease in the antioxidant activity with maturation of fruiting bodies (Lactarius sp.) may be caused by their involvement in defense against aging processes. In nature, antibacterial fungal compounds are supposed to protect fruiting bodies from microbial infections and they were found useful in biotechnology (Barros et al., 2007a; 2007b). The results of our experiments have proved that F. velutipes fruiting bodies may have antibacterial and bacteriostatic activities against S. aureus. At an early stage of maturation, strain Fv11 was able to inhibit growth of S. aureus completely at both tested concentrations (even in 2 x 106). The results obtained are similar to those of Barros et al. (2007a; 2007b), who indicated that loss of antibacterial activity during maturation of fruiting bodies may be related to loss of antioxidant properties. However, some fungal species are unable to stop the growth of S. aureus (Ramesh and Pattar, 2010) completely. Camelini et al. (2005) has proved that the concentration of glucan in Agaricus brasiliensis is related to cap formation and the glucan concentration is high only in mature fruiting bodies (cap open). Considering the F. velutipes images and carbohydrate concentration (Table I), there may be a similar correlation on the second and third day, which may be useful information for harvesting fruiting bodies without the need for complicated tests. In conclusion, the results obtained prove that Polish strains of Enoki have nutritional and nutraceutical values as edible mushrooms. Moreover, the results of our experiments indicate that the age of fruiting bodies is important for their healing properties.
Acknowledgments
This research was supported by the research program BS/UMCS.
Literature
Adebayo E.A., J.K. Oloke, D.A. Aina and T.C. Bora. 2014. Antioxidant and nutritional importance of some Pleurotus species J. Microbiol. Biotech. Food. Sci. 3:289-294.
Baminger U., S.S. Subramaniam, V. Renganathan and D. Haltrich. 2001. Purification and characterization of cellobiose dehydrogenase from the plant pathogen Sclerotium (Athelia) rolfsii. Appl. Environ. Microbiol. 67:1766-1774.
Barros L., P. Baptista, L.M. Estevinho and I.C. Ferreira. 2007a. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food. Chem. 55:8766-8771.
Barros L., P. Baptista and I.C.F.R. Ferreira. 2007b. Effect of Lactarius piperatus fruiting body maturity stage on antioxidant activity measured by several biochemical assays. Food Chem. Toxicol. 45:1731-1737.
Barros L., T. Cruz, P. Baptista, L.M. Estevinho and I.C. Ferreira. 2008. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 46:2742-2747.
Beyer Jr.W.F. and I. Fridovich. 1987. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 161:559-566.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
Cai H.H., X.M. Liu, Z.Y. Chen, S.T. Liao and Y.X. Zou. 2013. Isolation, purification and identification of nine chemical compounds from Flammulina velutipes fruiting bodies. Food Chem. 141:2873-2879.
Camelini C., M. Maraschin, M. de Mendonça, C. Zucco, A. Ferreira and L. Tavares. 2005. Structural characterization of β-glucans of Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical products. Biotechnol. Lett. 27:1295-1299.
Cao X., R. Liu, J. Liu, Y. Huo, W. Yang, M. Zeng and C. Yang. 2013. A novel polysaccharide from Lentinus edodes mycelia exhibits potential antitumor activity on laryngeal squamous cancer cell line Hep-2. Appl. Biochem. Biotechnol. 171:1444-1453.
Chang S.T. 1987. World production of cultivated edible mushrooms in 1986. Mushr. J. Tropics. 7:117–120.
Chang S.T. 2008. Overview of mushrooms cultivation and utilization as functional foods, pp. 1-33. In: Mushrooms as Functional Foods. John Wiley & Sons, Inc.
Chang Y.C., Y.M. Hsiao, M.F. Wu, C.C. Ou, Y.W. Lin, K.H. Lue and J.L. Ko. 2013. Interruption of lung cancer cell migration and proliferation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. J. Agric. Food. Chem. 61:12044-12052.
Cheung L.M., P.C.K. Cheung and V.E.C. Ooi. 2003. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 81:249-255.
Chiu K.W., A.H.W. Lam and P.K.T. Pang. 1995. Cardiovascular active substances from the straw mushroom, Volvariella volvacea. Phyt. Res. 9:93-99.
Deepalakshmi K., S. Mirunalini, M. Krishnaveni and V. Arulmozhi. 2013. In vitro and in vivo antioxidant potentials of an ethanolic extract of Ganoderma lucidum in rat mammary carcinogenesis. Chin. J. Nat. Med. 11:621-627.
Di Naso F.C., R.N. de Mello, S. Bona, A.S. Dias, M. Porawski, B. Ferraz Ade, M.F. Richter and N.P. Marroni. 2010. Effect of Agaricus blazei Murill on the pulmonary tissue of animals with streptozotocin-induced diabetes. Exp. Diabetes. Res. 2010:543-926.
DuBois M., K.A. Gilles, J.K. Hamilton, P.A. Rebers and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356.
Eisele N., D. Linke, K. Bitzer, S. Na’amnieh, M. Nimtz and R.G. Berger. 2011. The first characterized asparaginase from a basidiomycete, Flammulina velutipes. Bioresour. Technol. 102:3316-3321.
Ergonul P.G., I. Akata, F. Kalyoncu and B. Ergonul. 2013. Fatty acid compositions of six wild edible mushroom species. Scientific World J. 1-4.
Garcia M.X., H. Alexander, D. Mahadeo, D.A. Cotter and S. Alexander. 2003. The Dictyostelium discoideum prespore-specific catalase B functions to control late development and to protect spore viability. Biochim. Biophys. Acta. 1641:55-64.
Gunawardena D., L. Bennett, K. Shanmugam, K. King, R. Williams, D. Zabaras, R. Head, L. Ooi, E. Gyengesi and G. Munch. 2014. Anti-inflammatory effects of five commercially available mushroom species determined in lipopolysaccharide and interferon-gamma activated murine macrophages. Food Chem. 148:92-96.
Han Z.H., J.M. Ye and G.F. Wang. 2013. Evaluation of in vivo antioxidant activity of Hericium erinaceus polysaccharides. Int. J .Biol. Macromol. 52:66-71.
Hope C.F.A. and R.G. Burns. 1987. Activity, origins and location of cellulases in a silt loam soil. Biol. Fer. Soil. 5:164-170.
Iketani A., M. Nakamura, Y. Suzuki, K. Awai and Y. Shioi, 2013. A novel serine protease with caspase- and legumain-like activities from edible basidiomycete Flammulina velutipes. Fungal. Biol. 117:173-181.
Janes D., A. Umek and S. Kreft. 2006. Evaluation of antibacterial activity of extracts of five species of wood-colonizing fungi. J. Basic. Microbiol. 46:203-207.
Janusz G., A. Czuryło, M. Frąc, B. Rola, J. Sulej, A. Pawlik, M. Siwulski and J. Rogalski. 2014. Laccase production and metabolic diversity among Flammulina velutipes strains. World J. Microbiol. Biotechnol. 31: 121-133.
Jaszek M., J. Żuchowski, K. Dajczak, M. Cimek, M. Grąz and K. Grzywnowicz. 2006. Lignolytic enzymes can act as a part of multiple response system to oxidative stress in white rot Basidiomycetes Fomes fomentarius and Tyromyces pubescens. Int. Biodeter. Biodegr. 58:168-175.
Jaszek M., M. Osinska-Jaroszuk, G. Janusz, A. Matuszewska, D. Stefaniuk, J. Sulej, J. Polak, M. Ruminowicz, K. Grzywnowicz and A. Jarosz-Wilkolazka. 2013. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed. Res. Int. 2013:ID 497492.
Kalac P. 2013. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food. Agric. 93:209-218.
Karaman M., E. Jovin, R. Malbasa, M. Matavuly and M. Popovic. 2010. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 24:1473-1481.
Karapetyan K.N., T.V. Fedorova, L.G. Vasil’chenko, R. Ludwig, D. Haltrich and M.L. Rabinovich. 2006. Properties of neutral cellobiose dehydrogenase from the ascomycete Chaetomium sp INBI 2-26(-) and comparison with basidiomycetous cellobiose dehydrogenases. J. Biotechnol. 121:34-48.
Kim J.K., S.H. Lim and H.W. Kang. 2013. Cloning and characterization of a novel laccase gene, fvlac7, based on the genomic sequence of Flammulina velutipes. Mycobiology. 41:37-41.
Kotake T., N. Hirata, Y. Degi, M. Ishiguro, K. Kitazawa, R. Takata, H. Ichinose, S. Kaneko, K. Igarashi, M. Samejima and others. 2011. Endo-beta-1,3-galactanase from winter mushroom Flammulina velutipes. J. Biol. Chem. 286:27848-27854.
Kues U. and Y. Liu. 2000. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 54:141-152.
Lemieszek M. and W. Rzeski. 2012. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. (Pozn). 16:285-289.
Leonowicz A. and K. Grzywnowicz. 1981. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzyme Microb. Technol. 3:55-58.
Lin S.Y., Y.K. Chen, H.T. Yu, G.S. Barseghyan, M.D. Asatiani, S.P. Wasser and J.L. Mau. 2013. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 15:315-323.
Ma H., X. Xu and L. Feng. 2014. Responses of antioxidant defenses and membrane damage to drought stress in fruit bodies of Auricularia auricula-judae. World J. Microbiol. Biotechnol. 30:119-124.
Ma J.Q., C.M. Liu, Z.H. Qin, J.H. Jiang and Y.Z. Sun. 2011. Ganoderma applanatum terpenes protect mouse liver against benzo(alpha)pyren-induced oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 31:460-468.
Mahmood Z.A., S.W. Ahmed, I. Azhar, M. Sualeh, M.T. Baig and S. Zoha. 2010. Bioactive alkaloids produced by fungi. I. Updates on alkaloids from the species of the genera Boletus, Fusarium and Psilocybe. Pak. J. Pharm. Sci. 23:349-357.
Malarczyk E. 1989. Transformation of Phenolic-Acids by Nocardia. Acta Microbiol. Pol. 38: 45-53.
Mau J.-L., C.-N. Chang, S.-J. Huang and C.-C. Chen. 2004. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 87:111-118.
Moon B. and Y.M. Lo. 2013. Conventional and novel applications of edible mushrooms in today’s food industry. J. Food Process. Preserv. 38(5):2146-2153.
Moradali M.F., H. Mostafavi, S. Ghods and G.A. Hedjaroude. 2007. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 7:701-724.
Niedermeyer T.H., U. Lindequist, R. Mentel, D. Gordes, E. Schmidt, K. Thurow and M. Lalk. 2005. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 68:1728-1731.
Nyanhongo G.S., C. Sygmund, R. Ludwig, E.N. Prasetyo and G.M. Guebitz. 2013. An antioxidant regenerating system for continuous quenching of free radicals in chronic wounds. Eur. J. Pharm. Biopharm. 83:396-404.
Paduch R., G. Matysik, M. Wojciak-Kosior, M. Kandefer-Szerszen, A. Skalska-Kaminska, M. Nowak-Kryska and P. Niedziela. 2008. Lamium album extracts express free radical scavenging and cytotoxic activities. Pol. J. Environ. Stud. 17:569-580.
Pazdzioch-Czochra M., E. Malarczyk and J. Sielewiesiuk. 2003. Relationship of demethylation processes to veratric acid concentration and cell density in cultures of Rhodococcus erythropolis. Cell. Biol. Int. 27:325-336.
Rahman K. 2007. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2:219-236.
Ramesh C. and M.G. Pattar. 2010. Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacognosy Res. 2:107-112.
Re R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231-1237.
Santoyo S., A.C. Ramirez-Anguiano, L. Aldars-Garcia, G. Reglero and C. Soler-Rivas. 2012. Antiviral activities of Boletus edulis, Pleurotus ostreatus and Lentinus edodes extracts and polysaccharide fractions against Herpes simplex virus type 1. J. Food Nutr. Res. 51:225-235.
Sulej J., G. Janusz, M. Osinska-Jaroszuk, P. Malek, A. Mazur, I. Komaniecka, A. Choma and J. Rogalski. 2013. Characterization of cellobiose dehydrogenase and its FAD-domain from the ligninolytic basidiomycete Pycnoporus sanguineus. Enzyme Microb. Technol. 53:427-437.
Temp U. and C. Eggert. 1999. Novel interaction between laccase and cellobiose dehydrogenase during pigment synthesis in the white rot fungus Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 65:389-395.
Tong S., H. Zhong, C. Yi, X. Cao, C.K. Firempong, Q. Zheng, Y. Feng, J. Yu and X. Xu. 2014. Simultaneous HPLC determination of ergosterol and 22,23-dihydroergosterol in Flammulina velutipes sterol-loaded microemulsion. Biomed. Chromatogr. 28:247-254.
Wayne L.G. and G.A. Diaz. 1986. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 157:89-92.
Wiater A., R. Paduch, A. Choma, M. Pleszczynska, M. Siwulski, J. Dominik, G. Janusz, M. Tomczyk and J. Szczodrak. 2012. Biological study on carboxymethylated (1–>3)-alpha-D-glucans from fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 51:1014-1023.
Wright T. 2004. Medicinal mushrooms, pp: 26-29. In: Nutraceuticals World, Ramsey N.J. (ed.). Roman Publishing.
Wu G.S., J.J. Guo, J.L. Bao, X.W. Li, X.P. Chen, J.J. Lu and Y.T. Wang. 2013a. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum – a review. Expert Opin. Investig. Drugs. 22:981-992.
Wu S.J., T.M. Lu, M.N. Lai and L.T. Ng. 2013b. Immunomodulatory activities of medicinal mushroom Grifola frondosa extract and its bioactive constituent. Am. J .Chin. Med. 41:131-144.
Xu X., H. Yan, J. Chen and X. Zhang. 2011. Bioactive proteins from mushrooms. Biotechnol. Adv. 29:667-674.
Zhang Y., C. Venkitasamy, Z. Pan and W. Wang. 2013. Recent developments on umami ingredients of edible mushrooms – A review. Trends Food Sci. Technol. 33:78-92.
Zhang Z.F., G.Y. Lv, W.Q. He, L.G. Shi, H.J. Pan and L.F. Fan. 2013. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohyd. Polym. 98:1524-1531.
